Household heating and cooling systems follow the laws of thermodynamics.
Household heating and cooling systems follow the laws of thermodynamics.
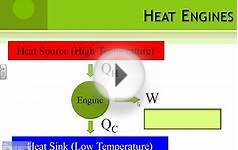
The laws of thermodynamics dictate energy behavior, for example, how and why heat, which is a form of energy, transfers between different objects. The first law of thermodynamics is the law of conservation of energy and matter. In essence, energy can neither be created nor destroyed; it can however be transformed from one form to another. The second law states that isolated systems gravitate towards thermodynamic equilibrium, also known as a state of maximum entropy, or disorder; it also states that heat energy will flow from an area of low temperature to an area of high temperature. These laws are observed regularly every day.
Melting Ice Cube
Every day, ice needs to be maintained at a temperature below the freezing point of water to remain solid. On hot summer days, however, people often take out a tray of ice to cool beverages. In the process, they witness the first and second laws of thermodynamics. For example, someone might put an ice cube into a glass of warm lemonade and then forget to drink the beverage. An hour or two later, they will notice that the ice has melted but the temperature of the lemonade has cooled. This is because the total amount of heat in the system has remained the same, but has just gravitated towards equilibrium, where both the former ice cube (now water) and the lemonade are the same temperature. This is, of course, not a completely closed system. The lemonade will eventually become warm again, as heat from the environment is transferred to the glass and its contents.
Sweating in a Crowded Room
The human body obeys the laws of thermodynamics. Consider the experience of being in a small crowded room with lots of other people. In all likelihood, you'll start to feel very warm and will start sweating. This is the process your body uses to cool itself off. Heat from your body is transferred to the sweat. As the sweat absorbs more and more heat, it evaporates from your body, becoming more disordered and transferring heat to the air, which heats up the air temperature of the room. Many sweating people in a crowded room, "closed system, " will quickly heat things up. This is both the first and second laws of thermodynamics in action: No heat is lost; it is merely transferred, and approaches equilibrium with maximum entropy.
Taking a Bath
Consider a situation where a person takes a very long bath. Immediately during and after filling up the bathtub, the water is very hot - as high as 120 degrees Fahrenheit. The person will then turn off the water and submerge his body into it. Initially, the water feels comfortably warm, because the water's temperature is higher than the person's body temperature. After some time, however, some heat from the water will have transferred to the individual, and the two temperatures will meet. After a bit more time has passed, because this is not a closed system, the bath water will cool as heat is lost to the atmosphere. The person will cool as well, but not as much, since his internal homeostatic mechanisms help keep his temperature adequately elevated.