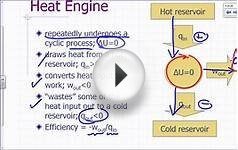
The second law of thermodynamics is based on our common human experience. It didn't begin with complicated apparatus or complex theories, but rather with thinking about how old-fashioned steam engines worked and the first important equation appeared to be very simple: just q/T.
Yet the second law is probably our most powerful aid in helping us understand why the world works as it does both in simple and in complex ways: why hot pans cool down, why ping pong balls don't bounce forever when they are dropped, why gasoline (plus the oxygen in air) makes engines run, why our "engines"—our bodies—run and we continue to live and our bodies stay warm even when it's cold, but also why we die when some chemical reactions within us fail. In fact, the second law helps to explain everything that happens in our physical world. In chemistry, it's especially important because it can tell us whether any chemical reaction that we write on paper will probably be spontaneous and go as we have written it.
The Big Problem[edit]
Unfortunately, for almost a century and a half, the second law has been expressed by experts in ways that a beginner in chemistry could not possibly understand without a great deal of additional explanation. Here are just three of some 25 explanations that have been most prominent:
- "The entropy of the universe increases toward a maximum" (Clausius)
- "It is impossible in any way to diminish the entropy of a system of bodies without thereby leaving behind changes in other bodies" (Planck)
- "In any irreversible process the total entropy of all bodies concerned is increased." (Lewis)
Entropy, entropy, entropy! But what is entropy? Even some textbooks still say something like "Don't ask about understanding it. Just work the problems that have entropy in them and you'll gradually understand it because you will be able to work with it"! That's the old way which fortunately has been discarded by most US general chemistry texts. (See list at .) The good news of the twenty-first century is that now entropy can be described as a simple idea (no matter how complex to calculate and deal with in advanced courses and research.) Because of our new conceptual approach, a basic version of the second law can be understood easily.








 Abiogenesis (pronounced /ˌeɪbaɪ.ɵˈdʒɛnɨsɪs/ AY-by-oh-JEN-ə-siss) or biopoiesis is the study of how biological life could arise from inorganic matter through natural processes. In particular, the term usually refers to the processes by which life on Earth may have...
Abiogenesis (pronounced /ˌeɪbaɪ.ɵˈdʒɛnɨsɪs/ AY-by-oh-JEN-ə-siss) or biopoiesis is the study of how biological life could arise from inorganic matter through natural processes. In particular, the term usually refers to the processes by which life on Earth may have...